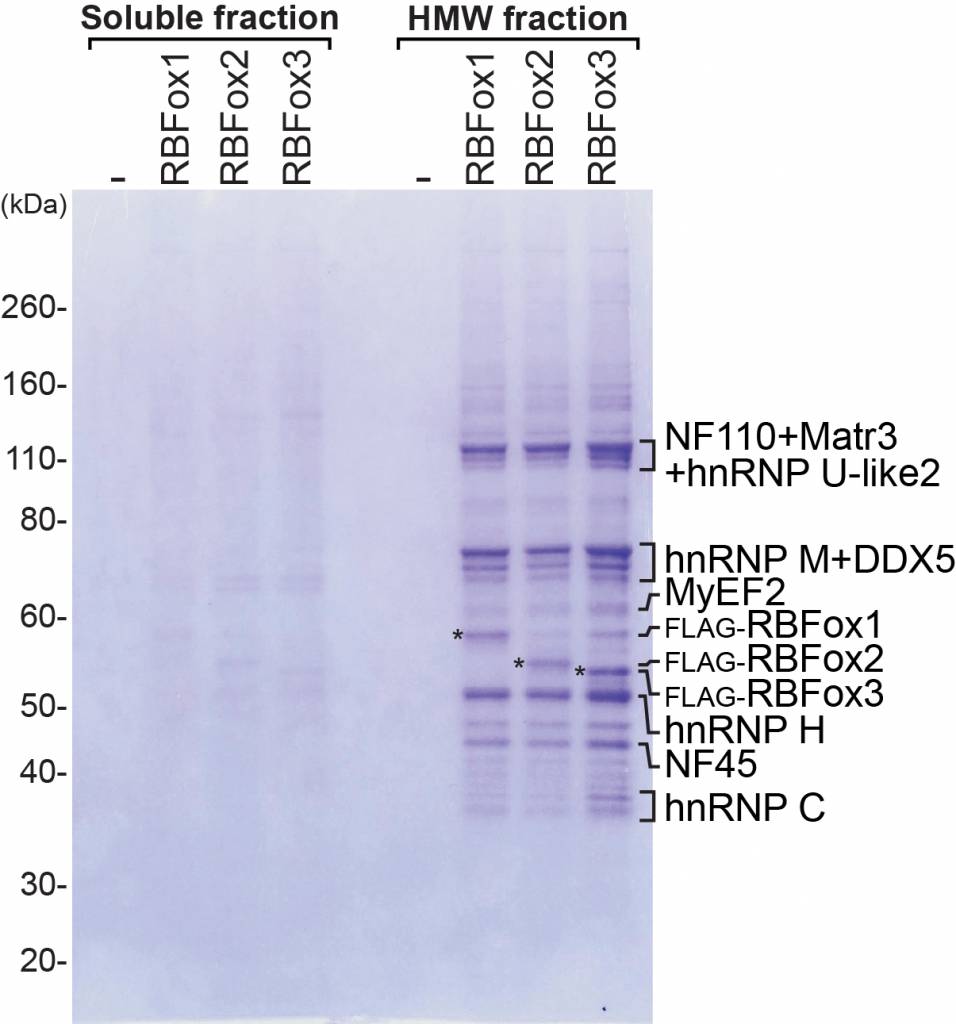
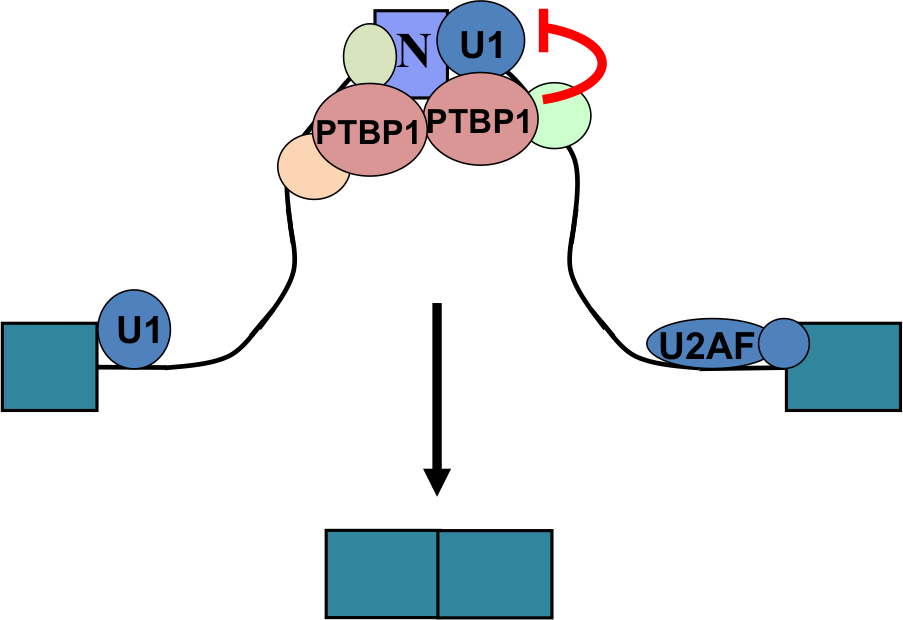
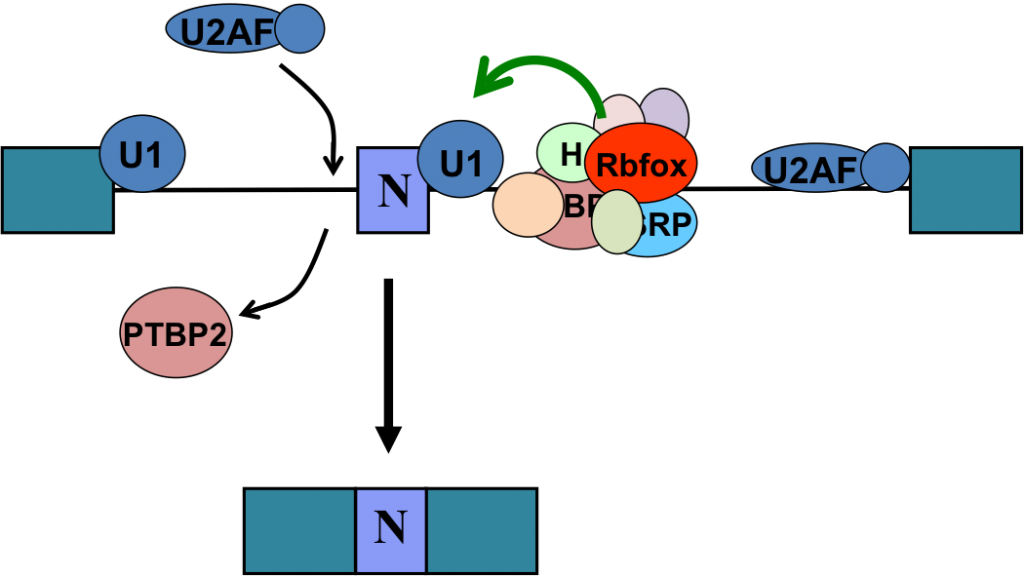
In mechanistic studies, we use biochemical methods, tissue culture, and in vitro splicing systems to examine the molecular interactions of splicing regulators and analyze how they can alter spliceosome assembly. We isolate exon complexes assembled onto regulated exons and characterize their architecture, using a variety of chromatographic, molecular crosslinking, and mass spectrometric methods. Following this approach, we have defined the interactions of PTBP1 with components of the spliceosome that mediate its repression activity. We have also shown that the Rbfox proteins engage with a novel higher order protein complex of multiple splicing regulators whose activity is determined by their interaction with Rbfox. Collaborating with structural biology groups, we work to define the structure of these proteins and their RNA binding domains.